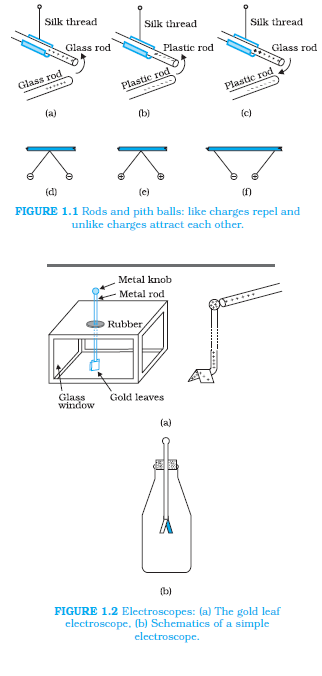
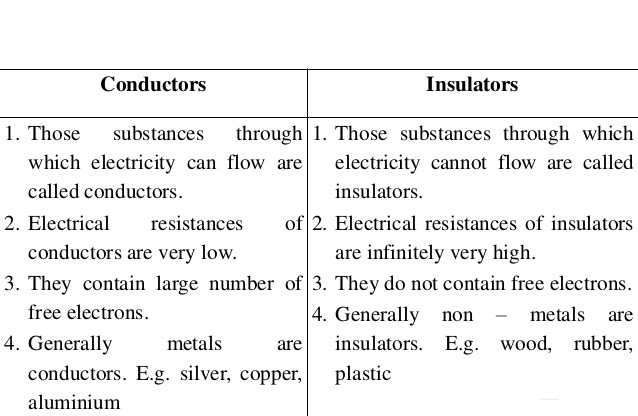
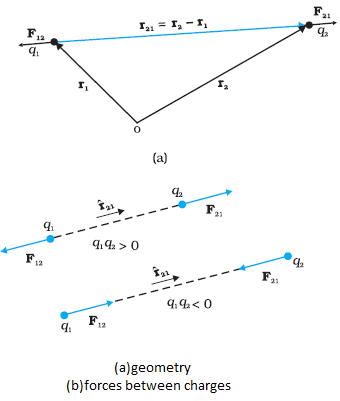
`\color{blue} ✍️` We have seen that there are two types of charges, namely positive and negative and their effects tend to cancel each other. Here, we shall now describe some other properties of the electric charge.
`\color{blue} ✍️` If the sizes of charged bodies are very small as compared to the distances between them, we treat them as point charges. All the charge content of the body is assumed to be concentrated at one point in space.
`color{purple}ulbb{"Additivity of charges : "}`
`\color{blue} ✍️` We have not as yet given a quantitative definition of a charge; we shall follow it up in the next section. We shall tentatively assume that this can be done and proceed.
`\color{blue} ✍️` If a system contains two point charges `q_1` and `q_2,` the total charge of the system is obtained simply by adding algebraically `q_1` and `q_2` , i.e., charges add up like real numbers or they are scalars like the mass of a body. If a system contains n charges `q_1, q_2, q_3, …, q_n`, then the total charge of the system is `q_1 + q_2 + q_3 + … + q_n .`
`\color{blue} ✍️` Charge has magnitude but no direction, similar to the mass. However, there is one difference between mass and charge. Mass of a body is always positive whereas a charge can be either positive or negative. Proper signs have to be used while adding the charges in a system. For example, the total charge of a system containing five charges `+1, +2, –3, +4` and `–5`,
in some arbitrary unit, is `(+1) + (+2) + (–3) + (+4) + (–5) = –1` in the same unit.
`color{purple}ulbb{"Charge is conserved : "}`
`\color{blue} ✍️` We have already hinted to the fact that when bodies are charged by rubbing, there is transfer of electrons from one body to the other; no new charges are either created or destroyed. A picture of particles of electric charge enables us to understand the idea of conservation of charge.
`\color{blue} ✍️` When we rub two bodies, what one body gains in charge the other body loses.
Within an isolated system consisting of many charged bodies, due to interactions among the bodies, charges may get redistributed but it is found that the total charge of the isolated system is always conserved.Conservation of charge has been established experimentally.
`\color{blue} ✍️` It is not possible to create or destroy net charge carried by any isolated system although the charge carrying particles may be created or destroyed in a process. Sometimes nature creates charged particles: a neutron turns into a proton and an electron. The proton and electron thus created have equal and opposite charges and the total charge is zero before and after the creation
`color{purple}ulbb{"Quantisation of charge "}`
`\color{blue} ✍️` Experimentally it is established that all free charges are integral multiples of a basic unit of charge denoted by `e.` Thus charge `q` on a body is always given by
`bb{q = n e}`
`\color{blue} ✍️` where n is any integer, positive or negative. This basic unit of charge is the charge that an electron or proton carries. By convention, the charge on an electron is taken to be negative; therefore charge on an electron is written as `–e` and that on a proton as `+e`.
`\color{blue} ✍️` The fact that electric charge is always an integral multiple of e is termed as quantisation of charge. There are a large number of situations in physics where certain physical quantities are quantised.
`\color{blue} ✍️` The quantisation of charge was first suggested by the experimental laws of electrolysis discovered by English experimentalist Faraday. It was experimentally demonstrated by Millikan in 1912.
`\color{blue} ✍️` In the International System (SI) of Units, a unit of charge is called a coulomb and is denoted by the symbol `C`. A coulomb is defined in terms the unit of the electric current which you are going to learn in a subsequent chapter. In terms of this definition, one coulomb is the charge flowing through a wire in 1 s if the current is 1 A (ampere), (see Chapter 2 of Class XI, Physics Textbook , Part I). In this system, the value of the basic unit of charge is `e = 1.602192 × 10^(–19) C`
`\color{blue} ✍️` Thus, there are about 6 × 1018 electrons in a charge of `–1C.` In electrostatics, charges of this large magnitude are seldom encountered and hence we use smaller units `1 μC` (micro coulomb) `= 10^(–6)` C or `1 mC` (milli coulomb) `= 10^(–3) C.`
If the protons and electrons are the only basic charges in the universe, all the observable charges have to be integral multiples of `e`.
`\color{blue} ✍️` Thus, if a body contains `n_1` electrons and `n_2` protons, the total amount of charge on the body is `n_2 × e + n_1 × (–e) = (n_2 – n_1) e.` Since `n_1` and `n_2` are integers, their difference is also an integer. Thus the charge on any body is always an integral multiple of e and can be increased or decreased also in steps of `e`
`\color{blue} ✍️` The step size `e` is, however, very small because at the macroscopic level, we deal with charges of a few `μC`. At this scale the fact that charge of a body can increase or decrease in units of e is not visible. The grainy nature of the charge is lost and it appears to be continuous. This situation can be compared with the geometrical concepts of points and lines.
`\color{blue} ✍️` A dotted line viewed from a distance appears continuous to us but is not continuous in reality. As many points very close to each other normally give an impression of a continuous line, many small charges taken together appear as a continuous charge distribution.
`\color{blue} ✍️` At the macroscopic level, one deals with charges that are enormous compared to the magnitude of charge e. Since `e = 1.6 × 10^(–19) C,` a charge of magnitude, say `1 μC`, contains something like `10^13` times the electronic charge. At this scale, the fact that charge can increase or decrease only in units of `e` is not very different from saying that charge can take continuous values.
`\color{blue} ✍️`Thus, at the macroscopic level, the quantisation of charge has no practical consequence and can be ignored. At the microscopic level, where the charges involved are of the order of a few tens or hundreds of `e`, i.e., they can be counted, they appear in discrete lumps and quantisation of charge cannot be ignored. It is the scale involved that is very important.
`\color{blue} ✍️` We have seen that there are two types of charges, namely positive and negative and their effects tend to cancel each other. Here, we shall now describe some other properties of the electric charge.
`\color{blue} ✍️` If the sizes of charged bodies are very small as compared to the distances between them, we treat them as point charges. All the charge content of the body is assumed to be concentrated at one point in space.
`color{purple}ulbb{"Additivity of charges : "}`
`\color{blue} ✍️` We have not as yet given a quantitative definition of a charge; we shall follow it up in the next section. We shall tentatively assume that this can be done and proceed.
`\color{blue} ✍️` If a system contains two point charges `q_1` and `q_2,` the total charge of the system is obtained simply by adding algebraically `q_1` and `q_2` , i.e., charges add up like real numbers or they are scalars like the mass of a body. If a system contains n charges `q_1, q_2, q_3, …, q_n`, then the total charge of the system is `q_1 + q_2 + q_3 + … + q_n .`
`\color{blue} ✍️` Charge has magnitude but no direction, similar to the mass. However, there is one difference between mass and charge. Mass of a body is always positive whereas a charge can be either positive or negative. Proper signs have to be used while adding the charges in a system. For example, the total charge of a system containing five charges `+1, +2, –3, +4` and `–5`,
in some arbitrary unit, is `(+1) + (+2) + (–3) + (+4) + (–5) = –1` in the same unit.
`color{purple}ulbb{"Charge is conserved : "}`
`\color{blue} ✍️` We have already hinted to the fact that when bodies are charged by rubbing, there is transfer of electrons from one body to the other; no new charges are either created or destroyed. A picture of particles of electric charge enables us to understand the idea of conservation of charge.
`\color{blue} ✍️` When we rub two bodies, what one body gains in charge the other body loses.
Within an isolated system consisting of many charged bodies, due to interactions among the bodies, charges may get redistributed but it is found that the total charge of the isolated system is always conserved.Conservation of charge has been established experimentally.
`\color{blue} ✍️` It is not possible to create or destroy net charge carried by any isolated system although the charge carrying particles may be created or destroyed in a process. Sometimes nature creates charged particles: a neutron turns into a proton and an electron. The proton and electron thus created have equal and opposite charges and the total charge is zero before and after the creation
`color{purple}ulbb{"Quantisation of charge "}`
`\color{blue} ✍️` Experimentally it is established that all free charges are integral multiples of a basic unit of charge denoted by `e.` Thus charge `q` on a body is always given by
`bb{q = n e}`
`\color{blue} ✍️` where n is any integer, positive or negative. This basic unit of charge is the charge that an electron or proton carries. By convention, the charge on an electron is taken to be negative; therefore charge on an electron is written as `–e` and that on a proton as `+e`.
`\color{blue} ✍️` The fact that electric charge is always an integral multiple of e is termed as quantisation of charge. There are a large number of situations in physics where certain physical quantities are quantised.
`\color{blue} ✍️` The quantisation of charge was first suggested by the experimental laws of electrolysis discovered by English experimentalist Faraday. It was experimentally demonstrated by Millikan in 1912.
`\color{blue} ✍️` In the International System (SI) of Units, a unit of charge is called a coulomb and is denoted by the symbol `C`. A coulomb is defined in terms the unit of the electric current which you are going to learn in a subsequent chapter. In terms of this definition, one coulomb is the charge flowing through a wire in 1 s if the current is 1 A (ampere), (see Chapter 2 of Class XI, Physics Textbook , Part I). In this system, the value of the basic unit of charge is `e = 1.602192 × 10^(–19) C`
`\color{blue} ✍️` Thus, there are about 6 × 1018 electrons in a charge of `–1C.` In electrostatics, charges of this large magnitude are seldom encountered and hence we use smaller units `1 μC` (micro coulomb) `= 10^(–6)` C or `1 mC` (milli coulomb) `= 10^(–3) C.`
If the protons and electrons are the only basic charges in the universe, all the observable charges have to be integral multiples of `e`.
`\color{blue} ✍️` Thus, if a body contains `n_1` electrons and `n_2` protons, the total amount of charge on the body is `n_2 × e + n_1 × (–e) = (n_2 – n_1) e.` Since `n_1` and `n_2` are integers, their difference is also an integer. Thus the charge on any body is always an integral multiple of e and can be increased or decreased also in steps of `e`
`\color{blue} ✍️` The step size `e` is, however, very small because at the macroscopic level, we deal with charges of a few `μC`. At this scale the fact that charge of a body can increase or decrease in units of e is not visible. The grainy nature of the charge is lost and it appears to be continuous. This situation can be compared with the geometrical concepts of points and lines.
`\color{blue} ✍️` A dotted line viewed from a distance appears continuous to us but is not continuous in reality. As many points very close to each other normally give an impression of a continuous line, many small charges taken together appear as a continuous charge distribution.
`\color{blue} ✍️` At the macroscopic level, one deals with charges that are enormous compared to the magnitude of charge e. Since `e = 1.6 × 10^(–19) C,` a charge of magnitude, say `1 μC`, contains something like `10^13` times the electronic charge. At this scale, the fact that charge can increase or decrease only in units of `e` is not very different from saying that charge can take continuous values.
`\color{blue} ✍️`Thus, at the macroscopic level, the quantisation of charge has no practical consequence and can be ignored. At the microscopic level, where the charges involved are of the order of a few tens or hundreds of `e`, i.e., they can be counted, they appear in discrete lumps and quantisation of charge cannot be ignored. It is the scale involved that is very important.